Abstract

BACKGROUND Chimeric antigen receptor (CAR) T-cell therapy for treatment of aggressive large B-cell non-Hodgkin lymphoma (B-NHL) has changed management and outcomes for this disease. Treatment with CAR T-cells is accompanied by a unique set of adverse effects, including development of cytokine release syndrome (CRS). Though rates of CRS varied in trials, the development of severe (Grade 3 or 4) CRS was rare and similar incidences have been noted in real world studies. Here, we addressed whether the development of CRS is associated with outcomes in patients with aggressive large B-NHL undergoing treatment with CAR T cells.
METHODS Patients aged ≥18 years with aggressive large B-NHL who underwent apheresis between May 2018 and January 2021 for commercial CAR T-cell therapy at eight academic US medical centers were identified from the Cell Therapy Consortium (CTC) registry. Providers could prescribe either axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel). CRS was graded using ASTCT consensus criteria. Tumor response 90 days after CAR T-cell infusion was assessed per Lugano criteria by the treating clinician. Persistent cytopenias were defined as ANC<1000 and/or Plt<50 and evaluated at the following intervals post infusion: day 30, 3 months, 6 months, 9 months, and one year. Cox regression analysis and the Kaplan Meier method were used for survival outcomes. Logistic regression analysis was used to assess association with tumor response and persistent cytopenias.
RESULTS This study included 351 patients who underwent apheresis for commercial CAR T cell therapy for aggressive large B-NHL (Table 1A). Of these, 262 (74.4%) developed CRS while 90 (25.5%) did not. The median age at apheresis was 61 (range 18-88) among patients who developed CRS and 65 (range 37-85) among those who did not (p=0.005). Of the patients included, 202 (57.6%) received axi-cel and 149 (42.5%) received tisa-cel.
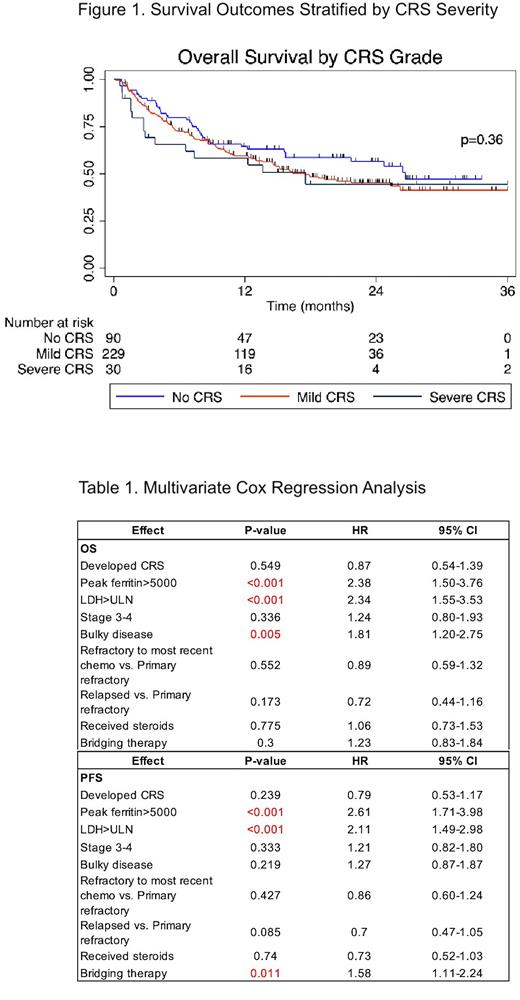
With median follow up of 21.6 months, there were no differences in progression free survival (PFS) or overall survival (OS) between patients who developed CRS and those who did not. Median PFS in patients who did not develop CRS was 5.99 months compared to 5.89 months (p=0.99) in those who did. Median OS in patients who did not develop CRS was 26.6 months compared to 17.5 months in patients who did (p=0.16). Exploratory analysis showed that CRS grade did not influence PFS or OS (Figure 1).
Results of the multivariate analysis are summarized in Table 1. The development of CRS did not have a significant impact on PFS or OS. A peak ferritin level of greater than 5000 within 28-days following CAR T-cell infusion was associated with worse PFS (HR 2.61; CI: 1.71-3.98, p<0.001) and OS (HR 2.38; CI: 1.50-3.76, p<0.001). LDH greater than the upper limit of normal was associated with worse progression-free (HR 2.11; CI: 1.49-2.98, p<0.001) and OS (HR 2.34; CI: 1.55-3.53, p<0.001). The administration of steroids for treatment of CAR T-cell associated toxicities had no significant impact on PFS or OS.
Presence of bulky disease (≥10 cm) had no impact on PFS but was associated with worse OS (HR 1.81; CI: 1.29-2.75, p=0.005). Receipt of bridging therapy was associated with worse PFS (HR 1.58; CI: 1.11-2.24, p=0.011), though not OS. At 30 days post-infusion, there was no difference in ORR (66% vs 52%, p=0.081) or CR rate (39% vs 27%, p=0.155) between patients who developed CRS and those who did not.
The occurrence of CRS within 28 days of treatment was significantly associated with cytopenias at day 30 (p=0.001), but not at any other time point. Amongst patients with Grade ≥3, 63% had cytopenias at day 30, compared to 47% among patients who did not have CRS (p=0.142). Grade ≥3 CRS was not significantly associated with cytopenias beyond day 30.
CONCLUSION Survival outcomes were similar in patients with aggressive large B-NHL treated with CAR T-cell therapy who did or did not develop CRS. Furthermore, the occurrence of CRS had no impact on ORRs or CR rates. Having CRS was associated with cytopenias at day 30 post infusion, though not at subsequent time points. These findings lend further support to the finding that development of CRS does not impact efficacy of CAR T-cell therapy.
Disclosures
Porter:Jazz: Consultancy; Adecept Bio: Consultancy; DeCART: Consultancy; BMS: Consultancy; Bluebird Bio: Consultancy; Kadmon: Consultancy; Angiocrine: Consultancy; Mirror Biologics: Consultancy; Genentech: Current equity holder in publicly-traded company; Roche: Current equity holder in publicly-traded company; Tmunity Therapeutics: Patents & Royalties: anti-CD19 CART; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Wiley: Honoraria; Elsevier: Honoraria; Janssen: Consultancy; Gerson Lerhman Group: Consultancy; Incyte: Consultancy; Kite/Gilead: Consultancy; Novartis: Consultancy, Patents & Royalties: anti-CD19 CART, Research Funding. Schuster:TG Therapeutics: Research Funding; Juno Therapeutics: Research Funding; DTRM: Research Funding; Adaptive Biotechnologies: Research Funding; Abbvie: Research Funding; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Nanovector: Consultancy; Nordic: Consultancy; MustangBio: Consultancy; Morphosys: Consultancy; Loxo: Consultancy; Legend Biotech: Consultancy; Janssen: Consultancy; Incyte: Consultancy, Research Funding; Genetech/Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; BiGene: Consultancy; Acerta: Consultancy; Merck: Research Funding; Pharmacyclics: Research Funding. Nastoupil:Genentech/Roche, MEI, Takeda: Other: DSMC; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria. Perales:Servier: Consultancy; VectivBio AG: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Omeros: Consultancy; Cidara Therapeutics: Consultancy; Sellas Life Sciences: Consultancy; Nektar Therapeutics: Consultancy, Honoraria; Vor Biopharma: Honoraria; Celgene: Honoraria; Orca Bio: Consultancy; MorphoSys: Consultancy, Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Karyopharm: Honoraria; Merck: Consultancy; Takeda: Honoraria; Medigene: Consultancy; Novartis: Honoraria; Bellicum: Honoraria; DSMB: Other; Incyte: Honoraria, Research Funding; Bristol-Mysers Squibb: Honoraria; Kite, a Gilead Company: Honoraria, Research Funding. Bishop:Tmunity: Research Funding; Triumvira: Research Funding; Immatics: Research Funding; Autolus: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; WindMIL Therapeutics: Consultancy; Bluebird Bio: Consultancy; Iovance: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria; ADC Therapeutics: Speakers Bureau; Sana Biotechnology: Consultancy; Chimeric Therapeutics: Consultancy; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Servier: Speakers Bureau. McGuirk:BMS: Consultancy, Honoraria, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; In8bio, Inc.: Other: IIT Clinical Trial; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Nextar: Consultancy, Honoraria; Orca Bio: Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; Novartis: Consultancy, Honoraria. Maziarz:Orca Bio: Other: Support for research analysis and for medical writing; ASTCT: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Support for research on CART; Allovir: Other: Support for research on Allo HCT costs of care of infectious related complications; CRISPR Therapeutics: Consultancy, Honoraria. Chen:Mesolbast: Honoraria; Morphosys: Honoraria. Bachanova:Citius Pharma: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; FATA Therapeutics: Research Funding; Incyte: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Karyopharma: Consultancy; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Riedell:Sana Biotechnology: Consultancy; Fate Therapeutics: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Calibr: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Research Funding. Oluwole:Novartis: Consultancy; Pfizer: Consultancy; TG Therapeutics: Consultancy; Curio Science: Consultancy; ADC Therapeutics: Consultancy; Janssen: Consultancy; Kite, a Gilead Company: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal